About Membership

※1 : Right to use MONSTAR-SCREEN during the study participation period for corresponding member companies.
※2 : Right to use MONSTAR-2 during the study participation period for corresponding member companies.
※3 : The service includes 1,500 cases in order of registration for GOZILA-STUDY participants.
※4 : The service includes 1-3,000 cases in order of registration for GOZILA-STUDY participants.
GOZILA-STUDY (3001 cases and beyond) and Circulate-Japan are also scheduled to be accessible.
About optional service
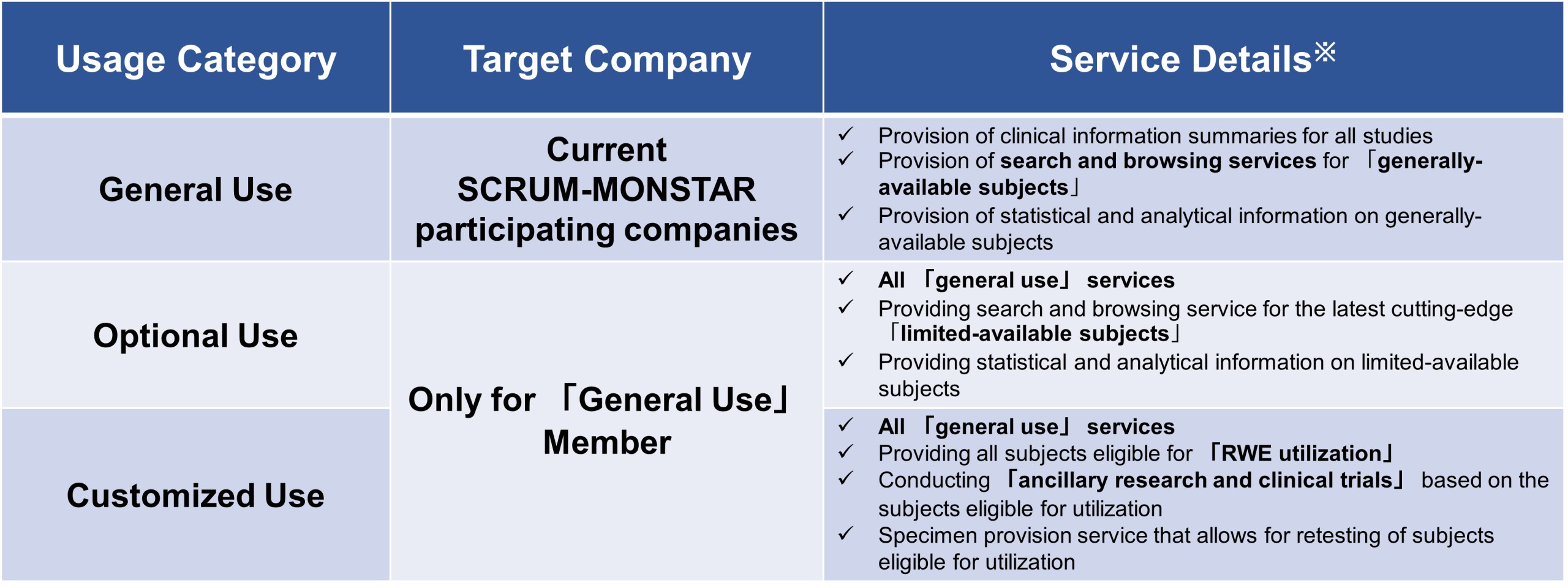
※: This service is limited to joint study data. But SCRUM-related clinical trial data is restricted to the implementing company.
「Generally-available subjects」refer to legacy data from studies after the end of the study, and the usage period is 5 years after the end of the study.
「Limited-available subjects」include「generally-available subjects」+「long-term follow-up data」, etc..
「Utilization subjects」 refer to all commercially necessary while available data (「limited-available subjects」 + samples + α).
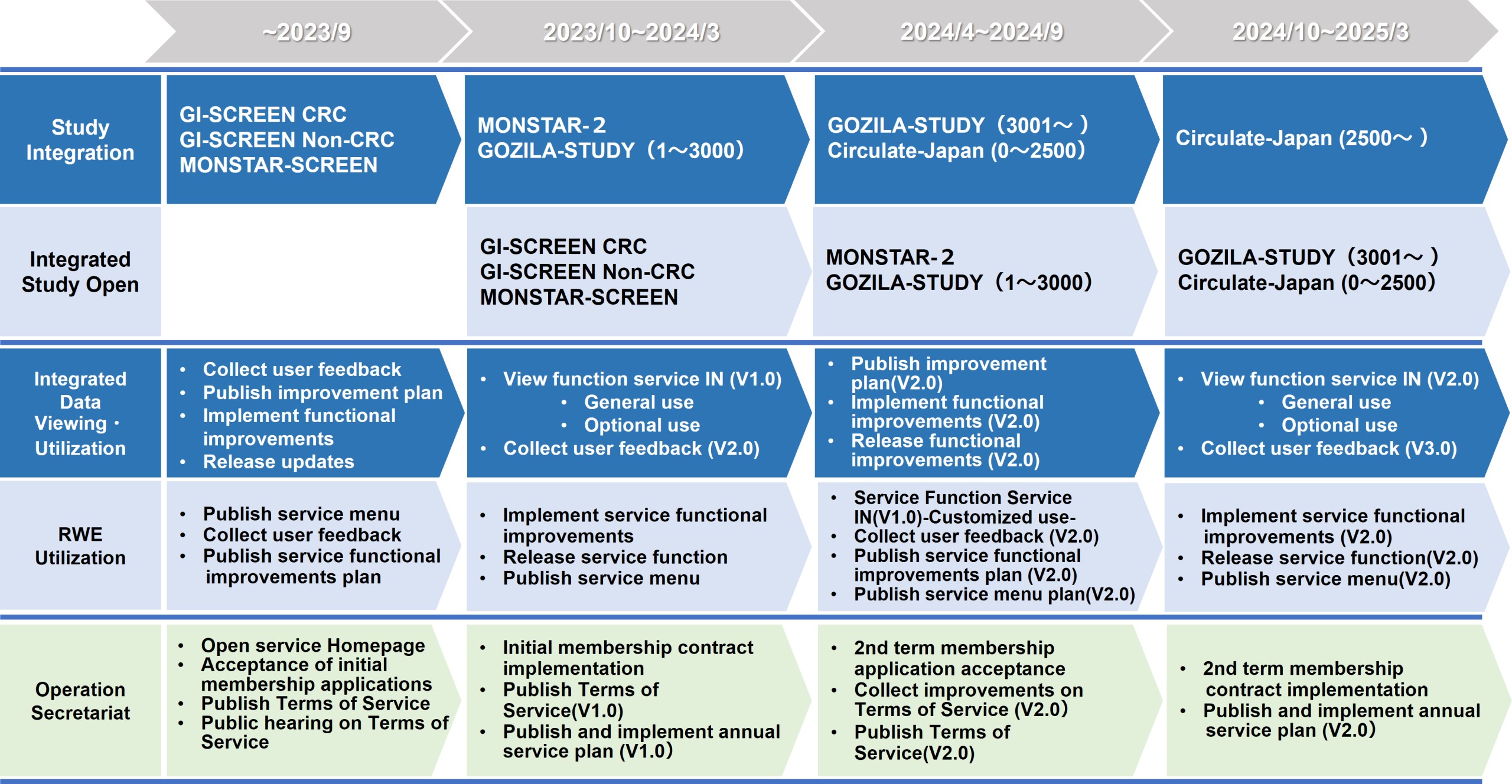
About roadmap